A Simple Guide to ePrescribe (eRx): The Pharmacy Informatics Fundamentals
ePrescribe is short for electronic prescriptions and is a method for providers to place medication orders to be electronically sent to community pharmacies for filling and dispensing. It is a form of CPOE (Computerized Provider Order Entry) for outbound prescribing. In order for ePrescribe to work, there are many technological aspects that must be followed. The following article is about the fundamentals of ePrescribe.
Key Terms
- ARRA: American Recovery and Reinvestment Act
- DEA: Drug Enforcement Administration
- EPCS: electronic prescriptions for controlled substances
- eRx / ePrescribe: electronic prescriptions
- HITECH: Health Information Technology for Economic and Clinical Health Act
- NCPDP: National Council for Prescription Drug Programs
Why ePrescribe?
Electronic prescribing provides many benefits related to not only patient safety but also operational efficiency. Some of those benefits include:
- Eliminates incorrect transcribing of provider orders since orders are electronically entered
- Improves pharmacy efficiency by reducing amounts of calls for clarification on orders
- Enhances historical logging of patient medications since electronic prescribing is logged and kept in the database
- Ensures prescription orders are complete and not missing fields because of enforced fields required by the electronic prescribing service
- Allows the use of alerts since medication orders are now stored electronically with coded values
A Brief History
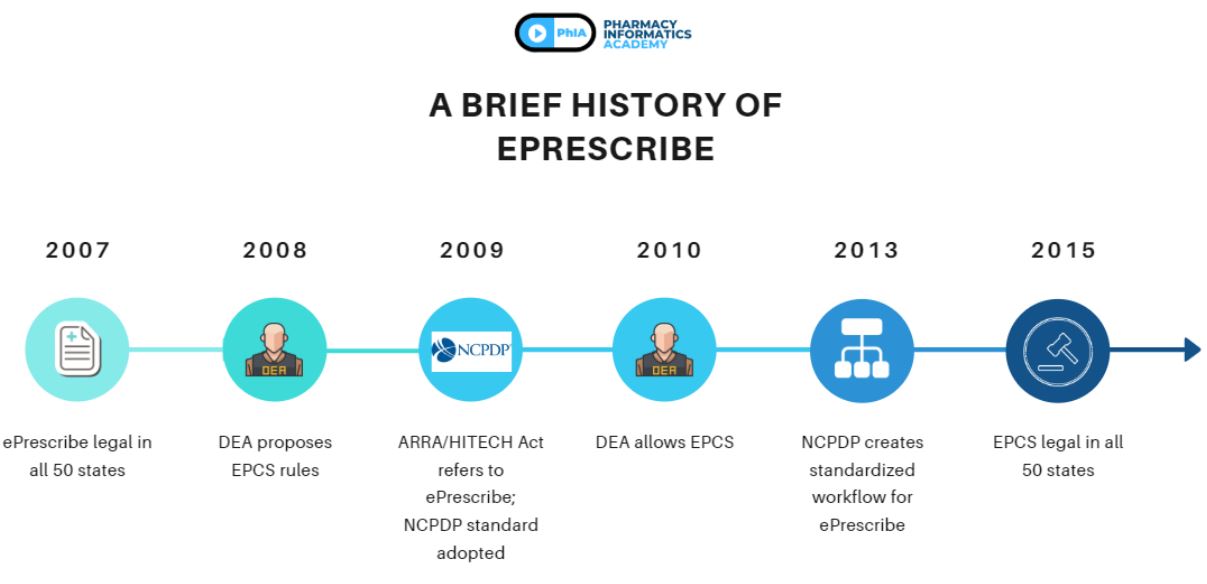
ePrescribe has gone through a number of steps to get to where it is today.
- 2007: Though ePrescribe has been talked about in as early as 2001, it was not until 2007 that ePrescribing became legal in all 50 states of the United States
- 2008: Because of the complicated processes behind authentication and liability, the DEA released proposed rules on how to ensure EPCS, or electronic prescribing of controlled substances, should be handled
- 2009: The ARRA/HITECH Act outlines what it means to ePrescribe, along with the eRx hub SureScripts adopting the NCPDP as a standard for ePrescribing
- 2010: The DEA finalizes and allows the use of EPCS
- 2013: After some years of ePrescribing and various lessons learned, the NCPDP created a workflow with the intent that it is the standardized workflow for all ePrescribing services
- 2015: EPCS becomes legal in all 50 states of the United States
High-Level Overview
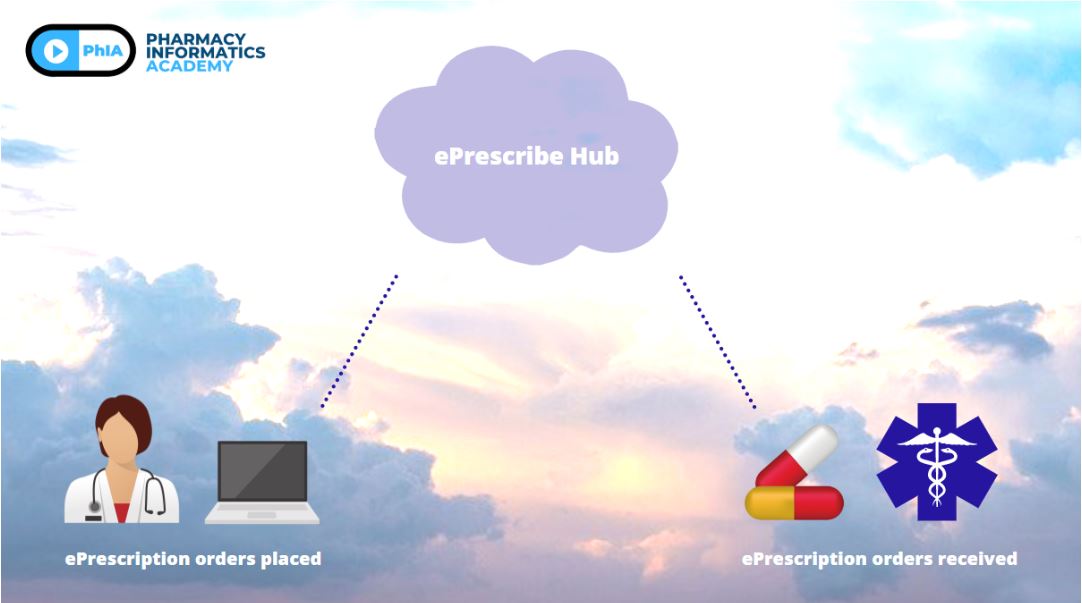
The fundamentals of ePrescribe start with a high-level overview. This diagram shows a high-level overview of a prescription order as it is placed through ePrescribe. The provider at the hospital or clinic would electronically place an order for a medication. This order is sent to a cloud ePrescribing hub for processing; once processing is complete and it meets certain requirements, it gets sent to the community pharmacy. The community pharmacy then processes this eRx at the pharmacy for filling and dispensing.
The ePrescribe Hub
In order for the provider’s orders to be understood by the community pharmacy, there needs to be a central processing hub that ensures the messages being received are in standardized format called the NCPDP Script Standard. In the United States, the ePrescribe Hub is called SureScripts and they have large amount of documentation on what’s required to be processed through their hub.
Institutions (Hospitals, Clinics, Providers)
The EHR that’s used at the clinic or organization must comply with all the necessary requirements for ePrescribe with SureScripts. As of today, most if not all EHR vendors in the United States meet this requirement. Configuration for ePrescribe is different for each EHR application and can include but is not limited to the following:
- Registration of a Surescripts Provider Identifier (SPI)
- Authentication of user
- Location and organization configuration
- Medication orders requiring fields that are necessary to be processed in the SureScripts hub
Pharmacies
Pharmacies that receive electronic prescriptions must use pharmacy software that meets the requirements of ePrescribe / SureScripts. This includes registration of the pharmacy within the SureScripts database with the appropriate identifiers, such as NPI, address, etc. This also includes certification of the software with SureScripts and the use of NCPDP Script Standard.
Electronic Prescribing of Controlled Substances (EPCS)
In addition to routine prescriptions, ePrescribe can also be used to order controlled substances. Additional layers of security and certifications are needed in order for EPCS to be enabled.
- Pharmacy software vendor must be certified through SureScripts and DEA-required audits
- EHR software vendor is certified and complies with requirements listed in DEA Rule
- Two-factor authentication is required for EPCS
- Provider must have an active DEA registration for controlled substance prescribing
As a reference, the DEA Rule states:
- DEA registrants are the only ones granted permission to sign controlled substance orders
- Method for EPCS must be strong enough to be trusted and valid
- EPCS must be highly reliable
- Security for EPCS must be able to prevent unauthorized controlled substance orders
Additional Resources
For additional resources on the fundamentals of ePrescribe, visit the following links:
Tips for Providers Starting E-Prescribing of Controlled Substances
E-Prescribing Quality Guidelines

March 24, 2020 @ 11:55 am
This is great! Would love to see more of these shorter, high level overview of various pharmacy informatics topics!
October 29, 2020 @ 12:28 pm
Thank you for your work, nicely done! At this time eRx(e-prescription) apps are developing rapidly. Besides, there are multifunctional apps that combine both EHR and eRx. According to Statista, 85 percent of all prescriptions in the US were made electronically. Undoubtedly, there is a big future in mobile healthcare apps.
November 16, 2020 @ 1:41 pm
Hello, thank you for your comment! There is definitely a big future in this as we integrate healthcare technology tools more into our lives.